03 March 2025 | Monday | News
Picture Courtesy | Public Domain
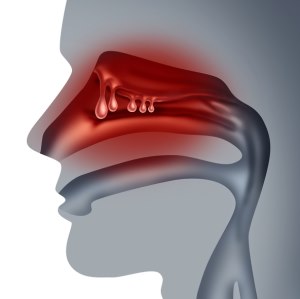
GSK plc announced full results from the positive ANCHOR-1 and ANCHOR-2 phase III clinical trials assessing the efficacy and safety of depemokimab versus placebo (both with standard of care [SOC]) in adults with CRSwNP. Depemokimab is an investigational, ultra-long-acting monoclonal antibody targeting interleukin-5 (IL-5), a key cytokine (protein) in type 2 inflammation that presents in up to 85% of people with CRSwNP. Results from these studies were presented in a late-breaking oral abstract session at the 2025 AAAAI/WAO Joint Congress in San Diego, California and simultaneously published in The Lancet.
ANCHOR-1 (N=271) and ANCHOR-2 (N=257) met their co-primary endpoints, with twice-yearly administration of depemokimab showing clinically meaningful and statistically significant improvements in nasal polyp size and nasal obstruction, two key clinical measures of disease severity, versus placebo. Additionally, a pooled analysis of the two trials showed improvements (reductions) from baseline versus placebo measured by:
ANCHOR-1 and ANCHOR-2 recruited a broad patient population with heterogenous symptom severity, reflective of real-world clinical practice. The treatment benefits were observed by the first assessment and sustained to week 52.
CRSwNP is a chronic condition that affects up to 4% of the general population. The current SOC, including surgery and SCS use, is suboptimal to address the long-term impact of CRSwNP, and almost half of patients live with poorly controlled symptoms. Although short-term SCS temporarily improves symptoms, repeated use is known to cause serious adverse events such as increased risk of diabetes, cardiovascular disease, cataracts and osteoporosis. Surgery also improves symptoms, but up to 40% of patients experience recurrence of nasal polyps and symptoms within 18 months due to the underlying inflammation not fully suppressed by surgery.
Findings from ANCHOR-1 and -2, along with data from SWIFT-1 and SWIFT-2, are being used to support regulatory filings in both asthma with type 2 inflammation and CRSwNP in different countries around the world. Depemokimab is not currently approved in any country for either of these indications.
© 2026 Biopharma Boardroom. All Rights Reserved.