18 February 2025 | Tuesday | News
Picture Courtesy | Public Domain
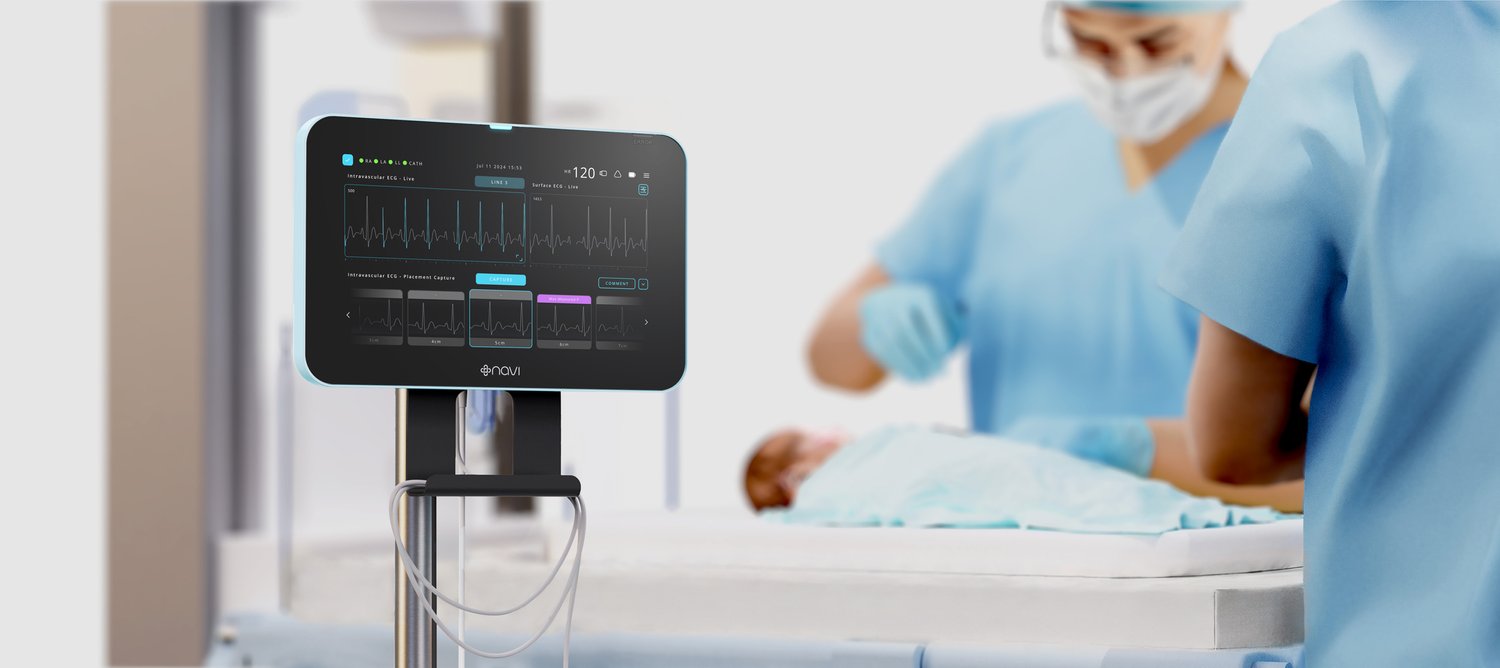
Navi Medical Technologies, a medical device company focused on pediatric healthcare innovation, proudly announces that its Neonav® ECG Tip Location System has received 510(k) clearance from the U.S. Food and Drug Administration (FDA).
This milestone represents a significant step forward in vascular access care for critically ill newborns and children worldwide. The Neonav® is the first medical device of its kind specifically designed for neonatal and pediatric care. By using real-time ECG signal analysis, it aids accurate placement of Central Venous Access Devices (CVADs), significantly reducing the risks associated with misplacement and migration which can cost US hospitals up to USD$1 Billion per year. This breakthrough innovation minimises reliance on confirmatory chest X-rays, will help in reducing delays in care, as well as lowering unintended complications for vulnerable patients.
The Neonav® system has the broadest 'Indication For Use' of any tip location system on the market, covering preterm newborns through to adults. Importantly, the system can be used to place tiny 1Fr catheters used in very small newborns, and is cleared for placement of catheters both above and below the heart. The system has also been developed to enable ongoing surveillance of catheters after the initial placement, an important first which can help to prevent potentially serious healthcare complications resulting from undiagnosed movement of the tip of the catheter. These novel features address critical challenges faced by healthcare professionals in neonatal and pediatric care.
© 2026 Biopharma Boardroom. All Rights Reserved.