05 May 2023 | Friday | News
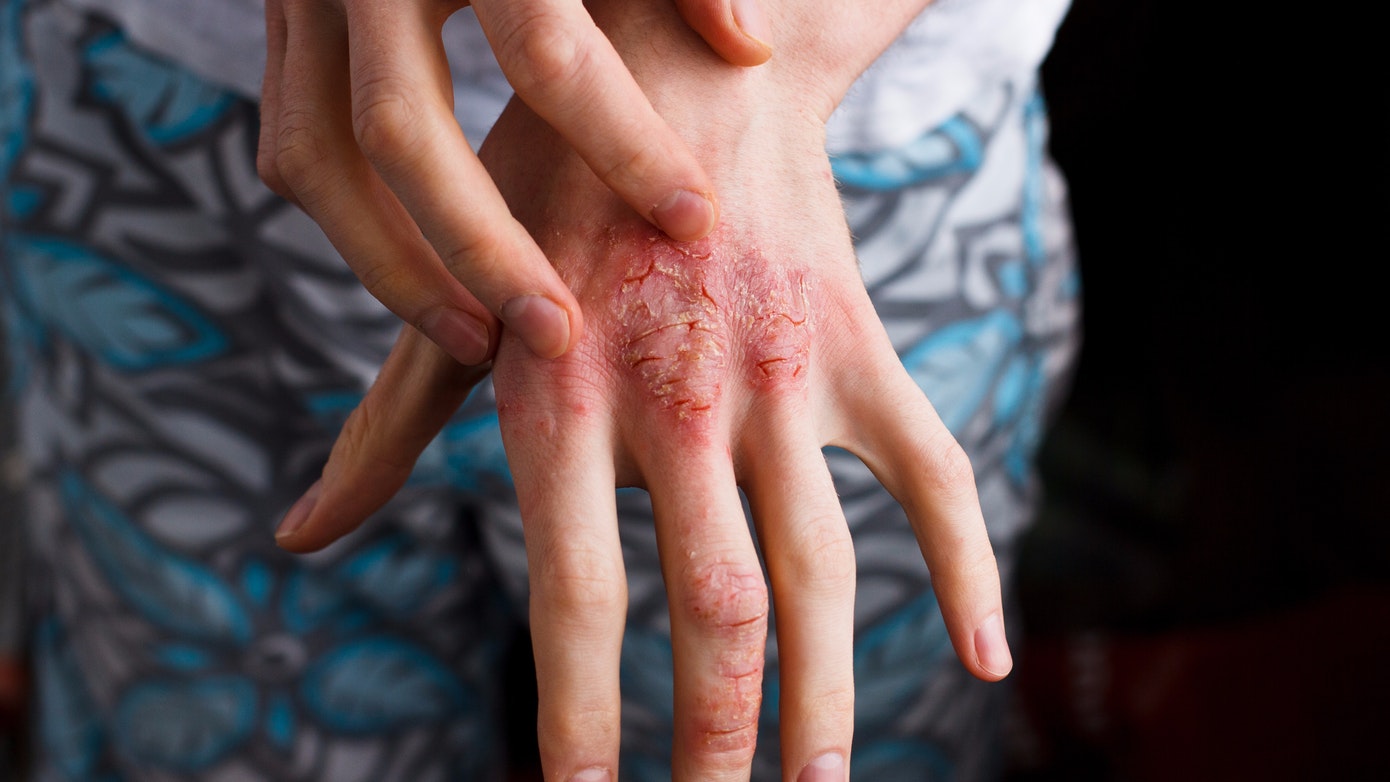
Alphyn Biologics, a clinical-stage dermatology company developing first-in-class multi-target therapeutics, announced positive results today from the pediatric population enrolled in its Phase 2a clinical trial of AB-101a, a novel topical candidate for mild-to-moderate atopic dermatitis (AD), at the 22nd European Society for Pediatric Dermatology Congress (ESPD). The first-of-its-kind trial met all primary and secondary efficacy endpoints in the pediatric population ages 2 and older with minimal safety and side effects.
AB-101a is in development to uniquely treat the immune and bacterial components of AD, with a safety profile that supports long-term continuous use. In January, Alphyn reported positive results of the first cohort of its Phase 2a trial. A second cohort of patients with AD evaluating AB-101a's impact on the bacterial complications of AD is ongoing.
"We are extremely pleased with the pediatric results announced today," said Alphyn CEO Neal Koller. "The improvements these children experienced in the severity of disease and symptoms are particularly remarkable. AD can be a life-long chronic disease, and options are needed for long-term continuous use."
The randomized double-blind Phase 2a trial is evaluating the safety and efficacy of AB-101a, with the first cohort of 41 patients ages 2 through adult, compared with a vehicle control. The pediatric data from the first cohort released today shows that all primary efficacy endpoints were met, including a reduction of disease severity indicated by the Investigator Global Assessment (IGA) score and minimal safety and side effects. Secondary endpoints of Eczema Area and Severity Index (EASI) score improvement, itch reduction immediately and long-term, and Body Surface Area (BSA) improvement were also achieved across all pediatric age groups. The pediatric results are detailed in a poster (P198) at ESPD.
Alphyn is developing AB-101a as the first therapeutic for AD that treats the immune system component and the bacterial complications of the disease, including those commonly associated with Staph (Staphylococcus aureus) and methicillin-resistant Staph (MRSA). Based on the positive trial results, Alphyn intends to initiate a multinational Phase 2b trial with sites in the United States, Europe, Canada, and Australia.
© 2026 Biopharma Boardroom. All Rights Reserved.