11 February 2025 | Tuesday | News
Picture Courtesy | Public Domain
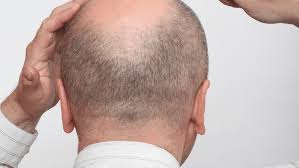
Q32 Bio Inc., a clinical stage biotechnology company focused on developing biologic therapeutics to restore immune homeostasis, announced a corporate restructuring to focus on the advancement of its bempikibart clinical development program for the treatment of patients with alopecia areata (AA). As part of the restructuring, the Company is discontinuing the Phase 2 renal basket clinical trial of ADX-097 and is evaluating strategic options for its tissue-targeted complement inhibitor platform, inclusive of ADX-097 and early-stage assets. In combination with other cost-saving measures including a reduction in personnel and related expenses, the strategic restructuring is expected to extend cash runway to the second half of 2026.
"We have conviction that bempikibart is differentiated from existing AA therapies and has the potential to transform the treatment paradigm for this disease. This is based on the continued emergence of bempikibart data in alopecia areata patients with longer-term follow-up from Part A of the SIGNAL-AA Phase 2a clinical trial, the robust pharmacologic data, and a well-tolerated safety profile," said Jodie Morrison, Chief Executive Officer of Q32 Bio. "Further, based on the clinical characteristics of bempikibart, we continue to believe there is potential utility across additional autoimmune conditions. Our immediate next steps will be to extend dosing of eligible patients from SIGNAL-AA Part A in an open-label extension arm and to initiate dosing of patients in the SIGNAL-AA Part B clinical trial in the first half of 2025, with Part B topline data expected in the first half of 2026."
Morrison added, "ADX-097, our Phase 2 complement inhibitor, is a clinical asset with strong potential to treat patients with complement disorders across multiple indications through its novel tissue-targeted approach. While this restructuring is a very difficult decision, it is a necessary step in the context of the evolving complement-mediated renal disease landscape and as we prioritize our focus and capital to maximize the potential of bempikibart. Our restructuring necessitates parting with some valued colleagues, and I want to personally thank them for their dedication to our mission, commitment to patients, and their many contributions to the Company."
© 2026 Biopharma Boardroom. All Rights Reserved.