23 January 2025 | Thursday | News
Picture Courtesy | Public Domain
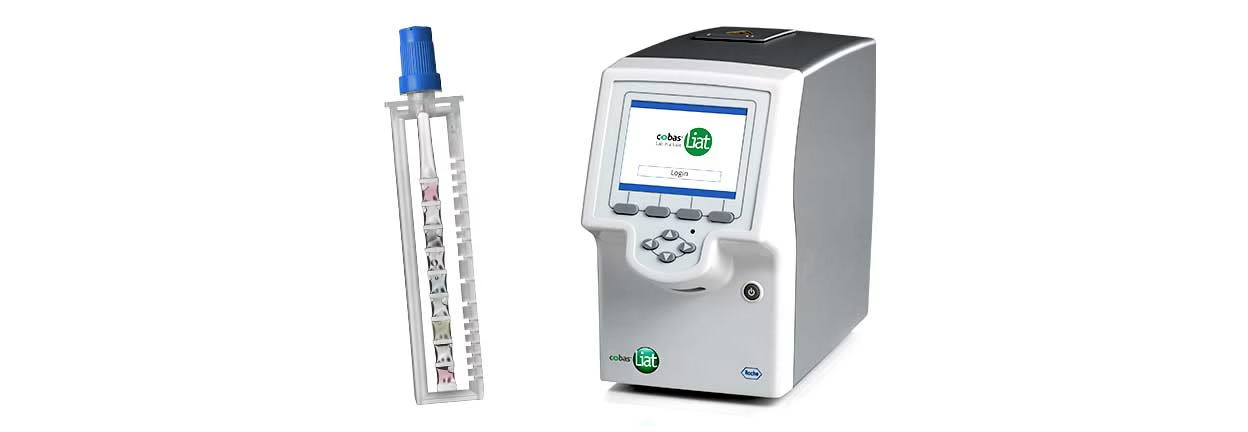
Roche (SIX: RO, ROG; OTCQX: RHHBY) announced that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance and Clinical Laboratory Improvement Amendments of 1988 (CLIA) waiver for its cobas® liat sexually transmitted infection (STI) multiplex assay panels. These panels, including tests for chlamydia and gonorrhea (CT/NG) and chlamydia, gonorrhea and Mycoplasma genitalium (CT/NG/MG), enable clinicians to diagnose and differentiate between multiple STIs with a single sample. These tests will be exclusively available in the U.S. market in the coming months, with commercialisation under CE mark expected to follow shortly.
“Rapid molecular point-of-care testing can revolutionise the clinical management of STIs in decentralised and community-based healthcare settings, enabling informed treatment strategies, better health outcomes for patients, and contain further spread by providing timely diagnosis.” said Matt Sause, CEO Roche Diagnostics.
More than 1 million people worldwide acquire an STI every day. Common STIs often present overlapping symptoms and can frequently be asymptomatic, making diagnosis challenging, when relying solely on symptoms. Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) are among the most prevalent STIs. If untreated, these infections can lead to serious health complications, including pelvic inflammatory disease (PID), urethritis, ectopic pregnancy, infertility, and an increased risk of HIV infection. Additionally, Mycoplasma genitalium (MG) is an emerging sexually transmitted pathogen affecting both males and females, with untreated infections resulting in severe health issues such as PID and infertility.
© 2026 Biopharma Boardroom. All Rights Reserved.