10 March 2025 | Monday | News
Picture Courtesy | Public Domain
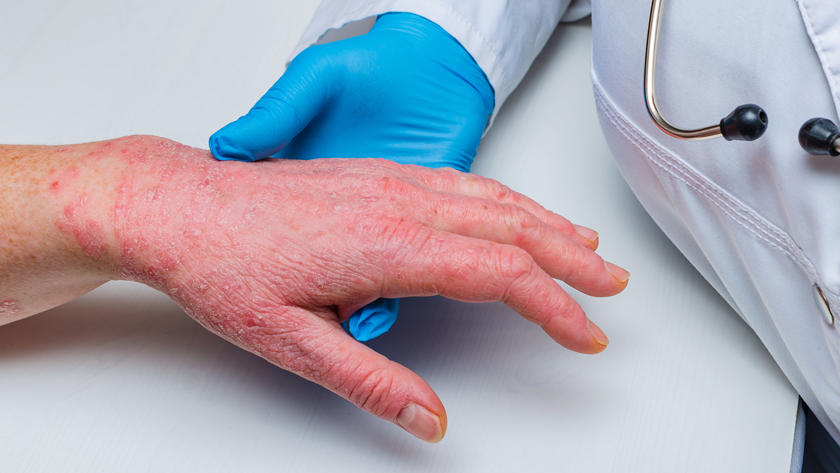
Johnson & Johnson announced new icotrokinra (JNJ-2113) data from its comprehensive Phase 3 clinical program and the start of the first-ever head-to-head study in plaque psoriasis (PsO) seeking to demonstrate the superiority of an oral pill, icotrokinra, compared to an injectable biologic, ustekinumab. Icotrokinra is a first-in-class investigational targeted oral peptide that selectively blocks the IL-23 receptor and is being studied in adults and adolescents 12 years of age and older with moderate-to-severe plaque PsO.
Data from the Phase 3 ICONIC-LEADa study, presented as a late-breaking abstract at the 2025 American Academy of Dermatology (AAD) Annual Meeting, show once daily icotrokinra demonstrated significant skin clearance and a favorable safety profile in adults and adolescents 12 years of age and older with moderate-to-severe plaque PsO.
In the ICONIC-LEAD study, nearly two-thirds (65%) of patients treated with once daily icotrokinra achieved an Investigator’s Global Assessment (IGA)b score of 0/1 (clear or almost clear skin) and 50% achieved a Psoriasis Area and Severity Index (PASI)c 90 response, compared to 8% and 4% receiving placebo, respectively (P<0.001 for both endpoints) at Week 16. Continued skin clearance improvement was reported at Week 24 with 74% of patients treated with icotrokinra achieving IGA 0/1 and 65% achieving PASI 90. At Week 24, nearly half of patients treated with icotrokinra achieved completely clear skin – 46% reached IGA 0 and 40% reached PASI 100. Similar proportions of patients experienced adverse events (AEs) between icotrokinra (49%) and placebo groups (49%), with no new safety signals identified.
Additionally, topline results show that the Phase 3 ICONIC-ADVANCE 1&2 studies met their co-primary endpoints of IGA 0/1 and PASI 90 versus placebo at Week 16. Icotrokinra also met all key secondary endpoints at Weeks 16 and 24 that measured superiority to deucravacitinib in patients with moderate-to-severe plaque PsO. Based on the positive outcomes of the ADVANCE studies, Johnson & Johnson is initiating the Phase 3 ICONIC-ASCEND study, the first-ever head-to-head study seeking to demonstrate the superiority of an oral pill, icotrokinra, compared to an injectable biologic, ustekinumab representing an important step forward in psoriasis research.
“The robust results seen to date underscore the potential for icotrokinra to shift treatment expectations in moderate-to-severe plaque psoriasis,” said Liza O’Dowd, Vice President, Immunodermatology Disease Area Lead, Johnson & Johnson Innovative Medicine. “As part of our ongoing commitment to pioneer innovations for patients, we are proud to advance this first-in-class investigational targeted oral peptide that selectively blocks the IL-23 receptor, which shows promise as a potential first-line systemic therapy for the treatment of plaque psoriasis.”
© 2026 Biopharma Boardroom. All Rights Reserved.