23 December 2025 | Tuesday | News
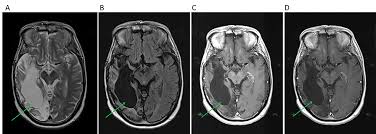
T-MAXIMUM Pharmaceutical announced that its proprietary allogeneic, B7-H3-targeted CAR-T therapy, MT027, has received IND Clearance from the U.S. Food and Drug Administration (FDA) to initiate a Phase II clinical trial for the treatment of recurrent glioblastoma (rGBM). This milestone marks a significant breakthrough in addressing one of the most formidable challenges in oncology: developing effective allogeneic CAR-T therapies for solid tumors.
"The FDA's clearance of the IND for MT027 represents a strong validation of our strategic commitment to tackling the most challenging solid tumors," said Dr. Xiaoyun Shang, Founder and CEO of T-MAXIMUM Pharmaceutical. "This milestone is not only a small step for T-MAXIMUM, but a significant leap forward for the entire cell therapy field as we push into the 'uncharted territory' of solid tumor treatment. The successful development and advancement of MT027 is grounded in our deep understanding of immunology and our decisive investment in allogeneic cell-editing technologies.
As a technology-driven company, T-MAXIMUM Pharmaceutical will continue to uphold a rigorous and pragmatic scientific approach, steadily advancing the clinical development of MT027. We remain committed to breaking new ground in the unexplored landscape of solid tumor cell therapy and using the power of science to win more time for patients."
© 2026 Biopharma Boardroom. All Rights Reserved.