13 February 2026 | Friday | News
Optune Pax concomitant with gemcitabine and nab-paclitaxel is the first treatment to be FDA approved in nearly 30 years for locally advanced pancreatic cancer
Phase 3 PANOVA-3 trial showed a statistically significant improvement in overall survival (OS) and significantly extended time to pain progression in patients treated with Optune Pax
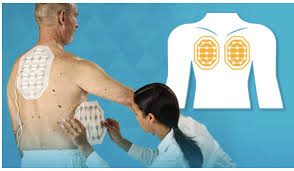
Optune Pax is a wearable medical device that delivers Tumor Treating Fields (TTFields), alternating electric fields that disrupt cancer cell replication to cause cell death, providing a new treatment approach for pancreatic tumors
Novocure (NASDAQ: NVCR) announced that the U.S. Food and Drug Administration (FDA) approved Optune Pax® for the treatment of adult patients with locally advanced pancreatic cancer concomitant with gemcitabine and nab-paclitaxel.
“In the Phase 3 PANOVA-3 trial, treatment with Optune Pax resulted in a statistically significant improvement in overall survival without adding to the systemic side effects commonly associated with existing therapies. It also significantly extended time to pain progression, helping to preserve overall quality of life, which is a priority when I am treating patients living with pancreatic cancer,” said Vincent Picozzi, M.D., MMM, medical oncologist and investigator in the PANOVA-3 trial. “With FDA approval, Optune Pax has the potential to be practice changing for the treatment of patients with locally advanced pancreatic cancer.”
Optune Pax is a portable therapeutic device that delivers Tumor Treating Fields (TTFields) non-invasively through wearable arrays. TTFields are alternating electric fields that target the electrical properties of cancer cells to disrupt processes critical for cancer cell division and survival, resulting in cell death without significantly affecting healthy cells.
“The FDA approval of Optune Pax marks the first new treatment in decades for people living with locally advanced pancreatic cancer. Systemic therapies have shown poor bioavailability in pancreatic tumors, limiting their effectiveness. Optune Pax is a fundamentally different treatment, utilizing a biophysical approach that targets the unique electrical properties of cancer cells,” said Frank Leonard, CEO, Novocure. “This is a proud moment for Novocure and we look forward to bringing Optune Pax to patients and the healthcare providers who care for them.”
“The approval of Optune Pax is an important milestone for the pancreatic cancer community. Survival rates for pancreatic cancer have seen only modest improvements over time and treatment advances have remained limited, underscoring how challenging this disease is to treat,” said PanCAN’s Chief Scientific and Medical Officer Anna Berkenblit, MD, MMSc. “This approval for locally advanced disease highlights the importance of continued innovation and investment in new approaches for difficult-to-treat cancers and represents meaningful progress for patients who urgently need more options.”
Data Supporting the Optune Pax FDA Approval
PANOVA-3 was an international, prospective, randomized, open-label, controlled Phase 3 clinical trial designed to evaluate the use of Optune Pax concomitantly with gemcitabine and nab-paclitaxel (gem/nab-pac) as a first-line treatment for locally advanced pancreatic cancer compared to gem/nab-pac alone.
The trial enrolled 571 patients who were randomized 1:1 and followed for a minimum of 18 months. The trial met its primary endpoint, demonstrating a statistically significant improvement in median overall survival (mOS) for patients treated with Optune Pax.
Optune Pax concomitant with gem/nab-pac demonstrated improvement in several secondary endpoints including the one-year survival rate.
Pancreatic cancer can cause significant pain as the disease progresses and managing pain is a key clinical challenge. In PANOVA-3, time to pain progression was defined as the time from baseline until an increase of 20 or more points was reported by patients on a visual scale for pain or until death.
Quality of life (QoL) was a secondary endpoint measured at baseline and every 8 weeks. Analyses were performed for all patients using the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) with the pancreatic cancer-specific PAN26 addendum. Treatment with Optune Pax concomitant with gem/nab-pac resulted in longer deterioration-free survival (DFS) in global health status, pain, pancreatic pain and most of the digestive problems. Similar trends were observed for emotional function and fatigue/lack of energy.
There was no significant difference in additional secondary outcome measures of progression-free survival, local progression-free survival, objective response rate, puncture-free survival or tumor resectability rate between the Optune Pax concomitant with gem/nab-pac and the gem/nab-pac alone arms.
Optune Pax was well-tolerated and did not exacerbate gem/nab-pac -related systemic toxicity, no new safety signals were observed, and serious adverse events (SAEs) were comparable between study arms. Most Optune Pax-treated patients experienced the expected device-related skin adverse events (AEs) under the arrays (76.3% of the Optune Pax-treated participants). The majority of these events were mild to moderate (Grade 1-2), with 21 (7.7%) experiencing a Grade ≥3 event. The most common device-related AE not related to skin adverse events was fatigue, reported in 14 participants (5.1%). There was one Grade 4 AE suspected to be related to the device by the investigator, which was a non-serious event of neutrophil count decrease. There were no device-related AEs that led to death, and no unanticipated device-related safety issues during the course of the study.
© 2026 Biopharma Boardroom. All Rights Reserved.