05 March 2025 | Wednesday | News
Picture Courtesy | Public Domain
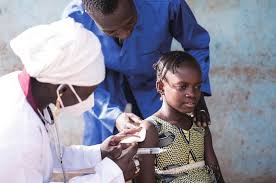
The Global Health Innovative Technology (GHIT) Fund is announcing a significant milestone: the first administration of arpraziquantel to preschool-aged children in Uganda, in an implementation science setting. This new pediatric treatment for schistosomiasis, developed by the GHIT-funded Pediatric Praziquantel Consortium, is being introduced through the Consortium's ADOPT program, which focuses on integrating arpraziquantel into existing healthcare platforms in countries where schistosomiasis is prevalent. This achievement marks GHIT's first supported innovation to reach people in need since its establishment in 2013.
"This isn't just a scientific achievement," said Osamu Kunii, CEO of the GHIT Fund. "It's a testament to the transformative power of global collaboration. By bringing together a diverse array of partners, each steadfastly contributing their unique expertise and sustaining momentum throughout a decade-long marathon of innovation to shepherd this breakthrough (from bench to bedside), arpraziquantel's development journey has unlocked solutions that once seemed impossible."
Schistosomiasis, or bilharzia, affects 250 million people globally, including an estimated 50 million preschool-aged children, mainly in sub-Saharan Africa. Left untreated, this poverty-related parasitic disease can lead to anemia, stunted growth, and impaired learning ability, as well as chronic inflammation of the organs, which can be fatal. Until now, a child-friendly treatment specifically tailored for preschool-aged children was not available, leaving millions of preschoolers at risk.
Early treatment of schistosomiasis in preschool-aged children is a crucial investment in public health and societal wellbeing. By intervening at this critical stage, we not only aim to shield young bodies from severe complications like organ damage and cognitive impairment but also alleviate the long-term burden on healthcare systems and hopefully better livelihoods.
The Pediatric Praziquantel Consortium developed arpraziquantel to address this gap. Japan's Astellas Pharma Inc., as a founding member of the Pediatric Praziquantel Consortium, played a pivotal role by utilizing its proprietary technology to lead arpraziquantel's initial formulation development, resulting in water dispersible, climate-stable, child-friendly tablets with acceptable taste. The formulation was optimized by Merck in Germany; the manufacturing process served to produce clinical trial supply from Merck and Farmanguinhos in Brazil. Current manufacturing is done by Farmanguinhos; future large-scale production by Universal Corporation Ltd. in Kenya is planned in and for Africa.
© 2026 Biopharma Boardroom. All Rights Reserved.