31 May 2023 | Wednesday | News
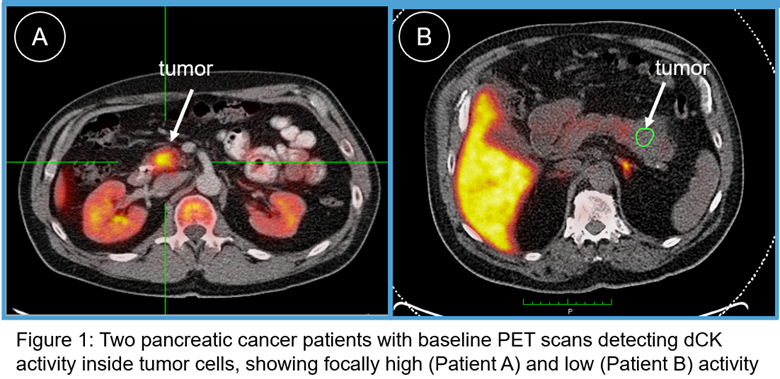
The grant will fund additional patient dosing studies to identify predictive biomarkers of activity and target engagement of TRE-515 in solid tumors. Trethera’s lead asset, TRE-515, is a first-in-class deoxycytidine kinase (“dCK”) inhibitor.
"We are honored to receive this highly competitive SBIR grant that supports additional clinical advancement and compelling opportunities for TRE-515," said Dr. Ken Schultz, principal investigator and Trethera CEO. "This grant allows further biomarker evaluations, helping to inform patient selection and dosing in order to target multiple types of difficult to treat cancers."
The NCI grant follows favorable results announced earlier this year from Trethera’s Phase 1a clinical trial in high risk, heavily pretreated patients with solid tumor malignancies. In the all comers designed (i.e., unselected) dose escalation trial, TRE-515 demonstrated a superb safety profile while also showing antitumor activity for 1 in 4 patients.
Dr. Tim Donahue, Trethera Board member and UCLA Chief of Surgical Oncology, commented, "With this funding, we intend to further showcase the therapeutic promise of disrupting nucleoside salvage pathway activity with TRE-515 as a late line therapy in solid tumors. We look forward to advancing TRE-515 through Phase 2 with the prospect of helping patients in need."
“The preliminary biomarker data support the underlying TRE-515 mechanism as an on-target dCK inhibitor that expects to positively impact disease progression in patients with solid tumors,” stated Dr. Jean DeKernion, Trethera Board member and UCLA Professor Emeritus of Urology. “A direct Phase 2 NCI award is incredibly competitive and a significant mark of distinction.”
A discussion summary from the panel of NCI experts that reviewed Trethera’s proposal stated, “the drug development plan is state of the art…noted efficacy in stabilizing disease progression in more than one patient in the early phase clinical trial…preliminary studies indicate that this assay can be validated…dCK represents an important and novel therapeutic target.”
TRE-515 is an orally delivered therapeutic engineered to inhibit dCK, the key enzyme in the nucleoside salvage pathway. A common characteristic of tumor cells in solid malignancies and pathological immune cells in autoimmune diseases is the requirement for elevated nucleotide levels to support abnormal and accelerated cell division. In contrast, dCK activity is not required in most healthy adult human cells. Mediated by the rate limiting enzyme, dCK, the nucleoside salvage pathway may play a pivotal role in enabling the rapid cell proliferation of cancer cells and aberrant activated lymphocytes, suggesting dCK as a potential therapeutic target with expected enhanced safety.
© 2026 Biopharma Boardroom. All Rights Reserved.