28 October 2024 | Monday | News
Picture Courtesy | Public Domain
The Cardiovascular Research Foundation's annual Transcatheter Cardiovascular Therapeutics (TCT) conference taking place at the Walter E. Washington Convention Center in Washington, DC, investigators from Gifu Heart Center and Fukuoka Sanno Hospital presented the results of the physician-initiated PROVISION Study. The investigators shared the first randomized controlled trial (RCT) of FFRangio outcomes compared to invasive wire-based fractional flow reserve (FFR) has met its primary endpoint and revealed significant economic and resource utilization advantages for the non-invasive FFRangio technology over traditional wire-based approaches.
The PROVISION Study included the enrollment of 400 patients across 13 centers in Japan. The principal investigators, Professor Hitoshi Matsuo from Gifu Heart Center and Professor Hiroyoshi Yokoi from Fukuoka Sanno Hospital, aimed to confirm that the physiological assessment with the wire-free angiography-based CathWorks FFRangio® System yields the same clinical treatment plans as those based on evaluation performed with invasive wire-based FFR, with no difference in their clinical prognoses. Additionally, the study aims to show that FFRangio offers economic advantages over wire-based FFR.
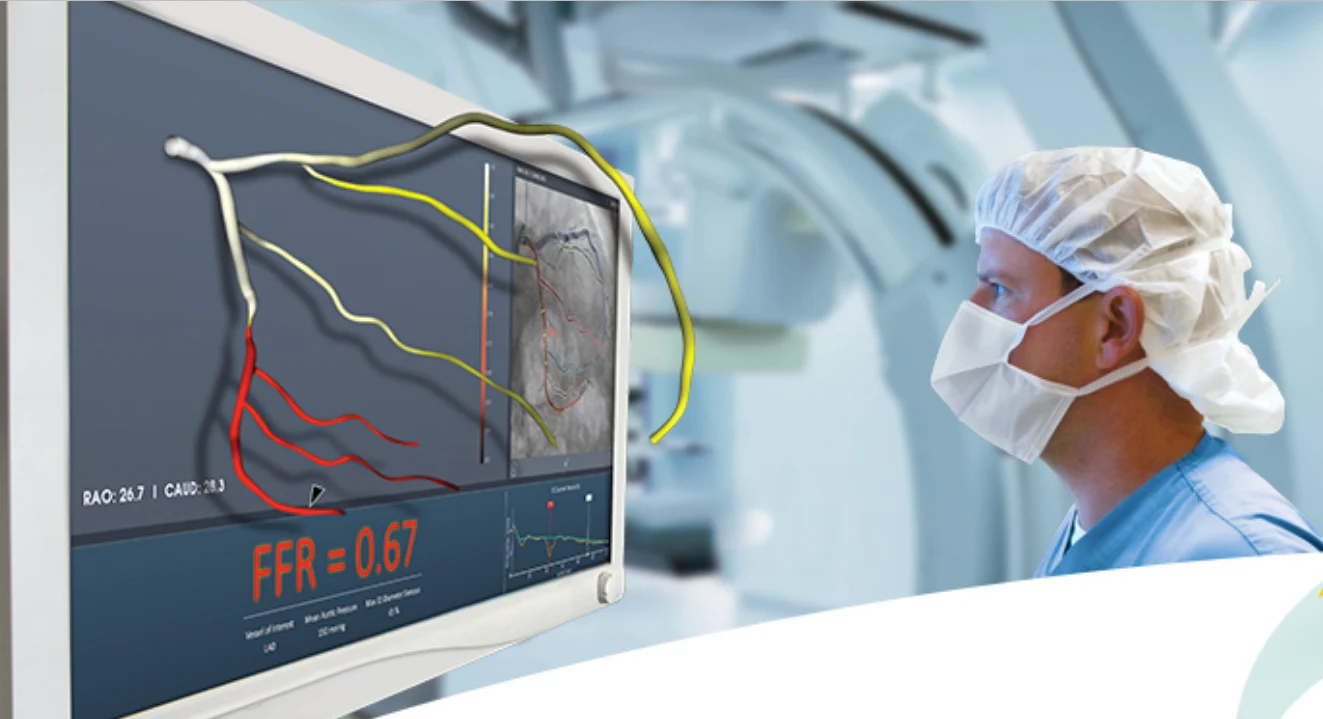
The FFRangio System combines artificial intelligence (AI) and advanced computational science, transforming how cardiovascular disease is diagnosed and treated. The FFRangio System obtains physiologic information from routine angiograms, eliminating the need for drug stimulation and invasive pressure wires. It provides physicians with quick and reliable intraprocedural FFRangio values for the entire coronary tree.
The PROVISION Study is the first Japanese prospective RCT comparing the outcomes of an angio-based physiology technology in comparison to invasive wire-based physiology. Patients with intermediate coronary lesions (30-90% diameter stenosis) were randomized 1:1 to either the wire-based FFR arm, in which the treatment decision was determined based on FFR values obtained using an invasive pressure wire, or the FFRangio arm, in which the treatment decision was based on FFRangio values. Based on the functional evaluations, either revascularization (PCI) and optimal medical management (OMT) or OMT alone were selected. For patients who underwent PCI, a post-PCI physiological assessment was performed using the respective technology, and patients will be followed clinically for at least one year.
Professor Yokoi shared, "The results from the post-PCI assessments performed on patients who underwent PCI and OMT, planned to be analyzed and reported after the completion of one-year follow-up, can provide the very much needed insight on optimal cut-off(s) for post-PCI FFR values, and provide the clinical evidence necessary to encourage routine post-PCI physiology assessment."
Professor Matsuo stated, "The results of this study are groundbreaking for coronary artery disease diagnostics in Japan. FFRangio offers a non-invasive alternative that not only matches the diagnostic accuracy of traditional wire-based FFR but also improves operational efficiencies and reduces costs for healthcare providers."
Key findings from the study include:
CathWorks President & CEO Ramin Mousavi added, "This significant milestone validates our mission of transforming how coronary artery disease is diagnosed and treatment through innovation. The results from the PROVISION Study have officially kicked off a new era in physiology, underscoring FFRangio as the new standard of care and potential to drive better patient outcomes while delivering tangible economic and resource utilization benefits to healthcare systems."
© 2026 Biopharma Boardroom. All Rights Reserved.