24 September 2025 | Wednesday | News
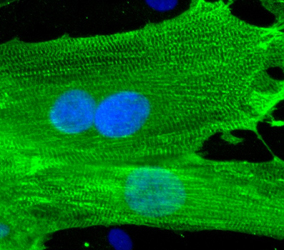
-Ncardia, a leader in stem cell–based solutions for drug discovery and safety assessment, announced the launch of Ncyte® NHP-C vCardiomyocytes, the first commercially available ventricular-like cardiomyocytes derived from induced pluripotent stem cells (iPSCs) of cynomolgus monkeys. New Ncyte® NHP-C vCardiomyocytes provide a scalable, ethical and translational tool for cross-species cardiac studies.
The innovation addresses a growing need for physiologically relevant, non-human primate (NHP) in vitro models that reduce reliance on live animals in preclinical research. With the launch of Ncyte® NHP-C vCardiomyocytes, Ncardia empowers the scientific community to conduct cross-species mechanistic studies, improve predictive modeling of cardiotoxicity and lower risk in drug development — all while advancing more ethical preclinical practices in alignment with the FDA recent animal testing guidance.
Ncyte® NHP-C vCardiomyocytes offer a high-purity, functional alternative that closely mimics human cardiac electrophysiology and pharmacology. The cells enable more predictive, scalable and ethical safety screening across species, supporting pharmaceutical and biotech companies in advancing safer therapies.
“As the only iPSC-derived NHP ventricular cardiomyocytes on the market, Ncyte® NHP-C vCardiomyocytes set a new standard for translational cardiovascular research,” said Jeroen de Groot, divisional chief executive of Ncardia. “They give researchers the ability to perform direct cross-species comparisons in vitro, improve cardiotoxicity risk assessment and meet the FDA’s evolving expectations around reducing the use of live non-human primates in safety testing.”
© 2026 Biopharma Boardroom. All Rights Reserved.