16 December 2025 | Tuesday | News
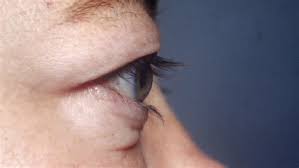
Minghui Pharmaceutical, a late-stage biopharmaceutical company dedicated to developing transformative medicines in immunology and oncology, today announced the U.S. Food and Drug Administration (FDA) has cleared its Investigational New Drug (IND) Application to initiate global Phase Ⅲ clinical trials of MHB018A for the treatment of thyroid eye disease (TED). The trials in both active and chronic TED are randomized, double-blind, placebo-controlled studies designed to evaluate the efficacy and safety of subcutaneous MHB018A administered every four weeks (Q4W).
MHB018A, a novel VHH-based IGF-1R antibody, is emerging as a potential best-in-class therapy, supported by compelling Phase Ⅱ data:
Detailed Phase Ⅱ results will be presented at upcoming medical conferences. The program was also highlighted at the 8th Evercore Healthcare Conference in December 2025.
"FDA Clearance of our Phase Ⅲ IND marks an important milestone and brings us a step closer to delivering a best-in-class therapeutic option to the large and underserved TED community." said Guoqing Cao, Ph.D., Chief Executive Officer of Minghui Pharmaceutical. "We are eager to advance MHB018A, our next-generation subcutaneous IGF-1R antibody with a differentiated profile. In China, we initiated a Phase Ⅲ trial in active TED in July 2025 and expect topline data in Summer 2026. We plan to begin enrollment of the Phase Ⅲ trial in chronic TED in early 2026. In the U.S., we anticipate initiating Phase Ⅲ trials in the first half of 2026. As these global efforts progress, our focus remains on moving MHB018A efficiently toward registration and ultimately providing a meaningful treatment option for patients living with TED."
© 2026 Biopharma Boardroom. All Rights Reserved.