30 May 2024 | Thursday | News
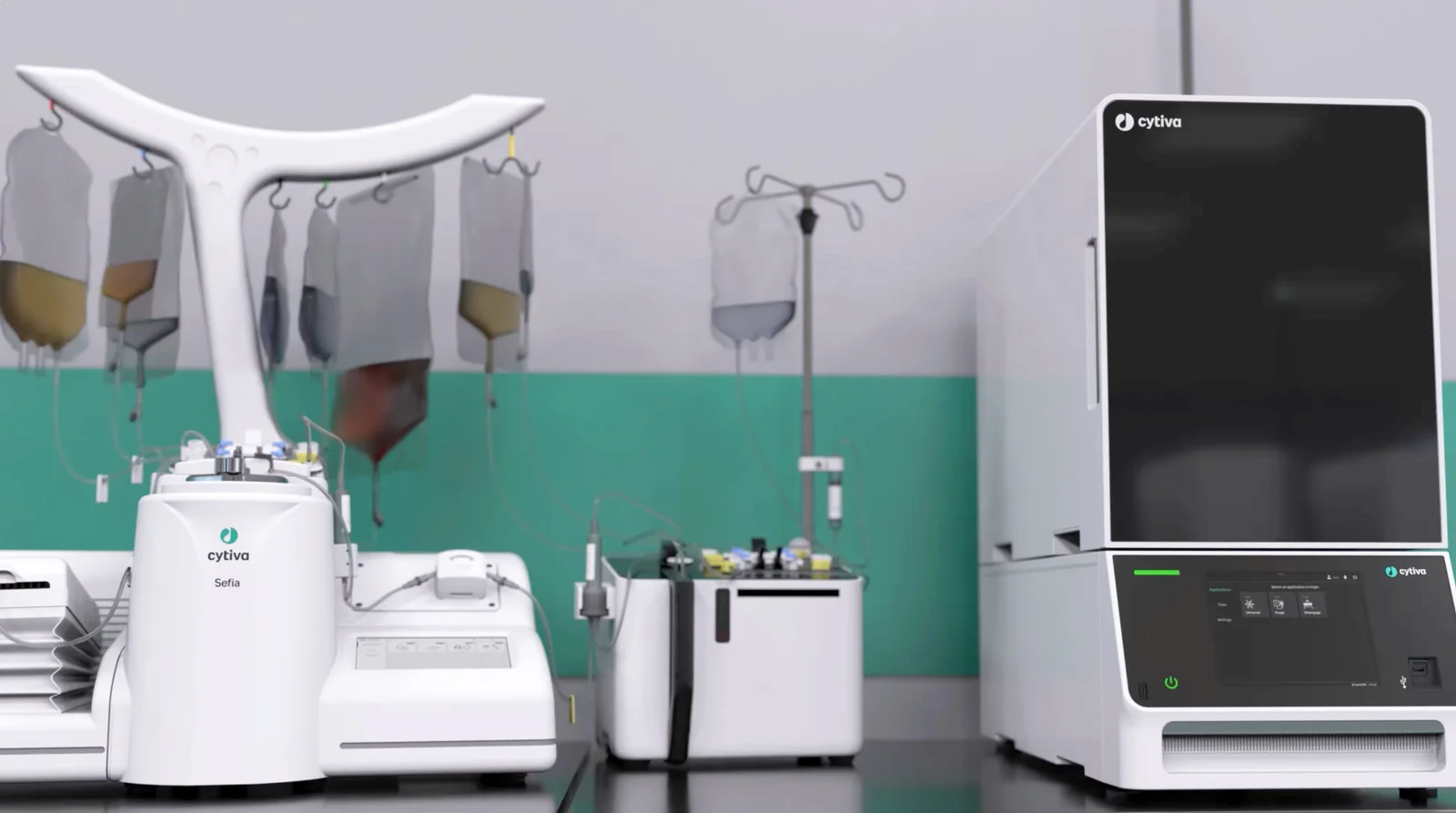
Cytiva is addressing industry challenges that hinder patient access and broader adoption of autologous CAR T cell therapies with the launch of the new Sefia cell therapy manufacturing platform. This latest innovation in the complete cell therapy workflow builds on Cytiva’s extensive experience in manufacturing advanced therapies. The Sefia platform was developed in collaboration with Kite, a Gilead Company, a global leader in autologous CAR T cell therapies.
Autologous CAR T cell therapies, due to their personalized nature, have historically relied heavily on manual labor, requiring product integrity maintenance in high-grade cleanrooms with skilled personnel. This complex manufacturing process, coupled with limited capacity and the potential risk of batch failures, has made it challenging for drug developers to meet the growing patient demand. Cytiva’s Sefia cell therapy manufacturing platform is specifically designed to overcome these challenges.
Emmanuel Abate, President of Genomic Medicine at Cytiva, states: “Our new manufacturing platform is the culmination of more than a decade of scientific research. Working hand-in-hand with industry leaders, we developed a platform designed to significantly increase productivity, helping ensure patients can have greater access to life-changing advanced therapies. It’s also a reflection of both Kite and Cytiva’s commitment to collaboration and accelerating the manufacture of autologous CAR T cell therapies.”
Currently, there are 10 autologous CAR-T cell therapies officially approved for use in the United States, the European Union, and the Asia-Pacific region. Additionally, there are over 1,000 cell therapy clinical trials underway globally, making flexible, scalable, and efficient manufacturing crucial to meeting patient demand.
The Sefia cell therapy manufacturing platform combines the Sefia Select system and the Sefia Expansion system. Utilizing a modular approach with two functionally closed and digitally integrated systems, key steps in the workflow are automated. The Sefia Select system automates the cell isolation, harvest, and formulation steps, while the Sefia Expansion system automates the cell activation, transduction, and cell expansion steps.
Automation eliminates many manual steps where human errors can increase manufacturing time and potentially cause cell stress. The platform can also be connected to Cytiva’s Chronicle automation software to monitor facility manufacturing operations and manage supply chain logistics.
Abate further explains: “Our goal has always been to help our customers scale-up the manufacture of these therapies while reducing risk factors. The Sefia platform was designed to help increase manufactured doses by up to 50% per year compared to industry standards, which can help ultimately reduce the cost burden.”
Cytiva customers benefit from best-in-class service offerings and dedicated teams, covering everything from basic repairs to specialized training programs for users, operators, and staff. Cytiva’s team can also assist with quality documentation to ensure regulatory compliance at every step of the manufacturing workflow.
© 2026 Biopharma Boardroom. All Rights Reserved.