02 October 2024 | Wednesday | News
Picture Courtesy | Public Domain
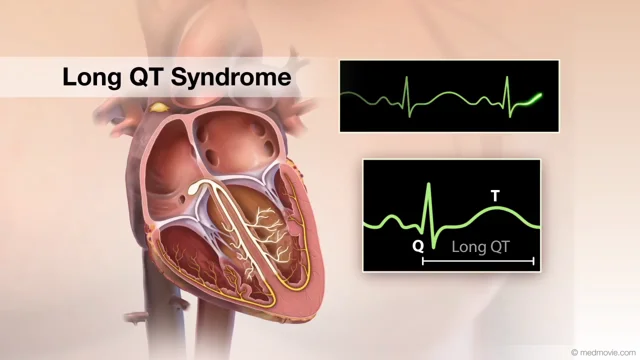
Thryv Therapeutics, a clinical stage biotech company dedicated to developing treatments for rare and life-threatening cardiovascular indications, announced that the United States Food and Drug Administration (FDA) has granted Orphan Drug Designation to LQT-1213 for the treatment of Long QT Syndrome (LQTS). LQT-1213 is a novel, first-in-class SGK1 inhibitor specifically designed to treat congenital LQTS. Thryv has developed a series of SGK1 inhibitors for the treatment of cardiometabolic stress associated with various arrhythmic diseases including LQTS, atrial fibrillation, and heart failure.
"We are pleased with the FDA's Orphan Drug Designation for LQT-1213 based partly on emerging positive clinical data from our Wave 1 clinical study in patients with Long QT Syndrome" said Debra Odink, PhD, President and Chief Development Officer of Thryv Therapeutics. "People with Long QT Syndrome deserve a properly studied and FDA-approved therapy to help in their battle against this potentially lethal genetic disease. There is currently no FDA-approved therapy for people with Long QT Syndrome. This designation reinforces the potential of LQT-1213 to fulfill this unmet need and provides critical incentives for Thryv to accelerate our efforts to deploy prospectively designed, pivotal efficacy studies in people with congenital Long QT Syndrome. We remain focused on bringing innovative treatments to adults and children with rare diseases who have limited options."
Following a request from the FDA, clinical data from the ongoing Wave I clinical study in people with congenital LQTS was provided to the Office of Orphan Products Designation. Shortly thereafter, the company received notification that its request for Orphan Drug Designation was approved.
The FDA Orphan Drug Designation is a special status granted to drugs and biologics targeting rare diseases, defined as those affecting fewer than 200,000 people in the United States. This designation offers numerous benefits to support the development of treatments for rare conditions, including tax credits for clinical trial costs, exemption from certain FDA fees, and up to seven years of market exclusivity following FDA approval.
© 2026 Biopharma Boardroom. All Rights Reserved.