27 January 2025 | Monday | News
Picture Courtesy | Public Domain
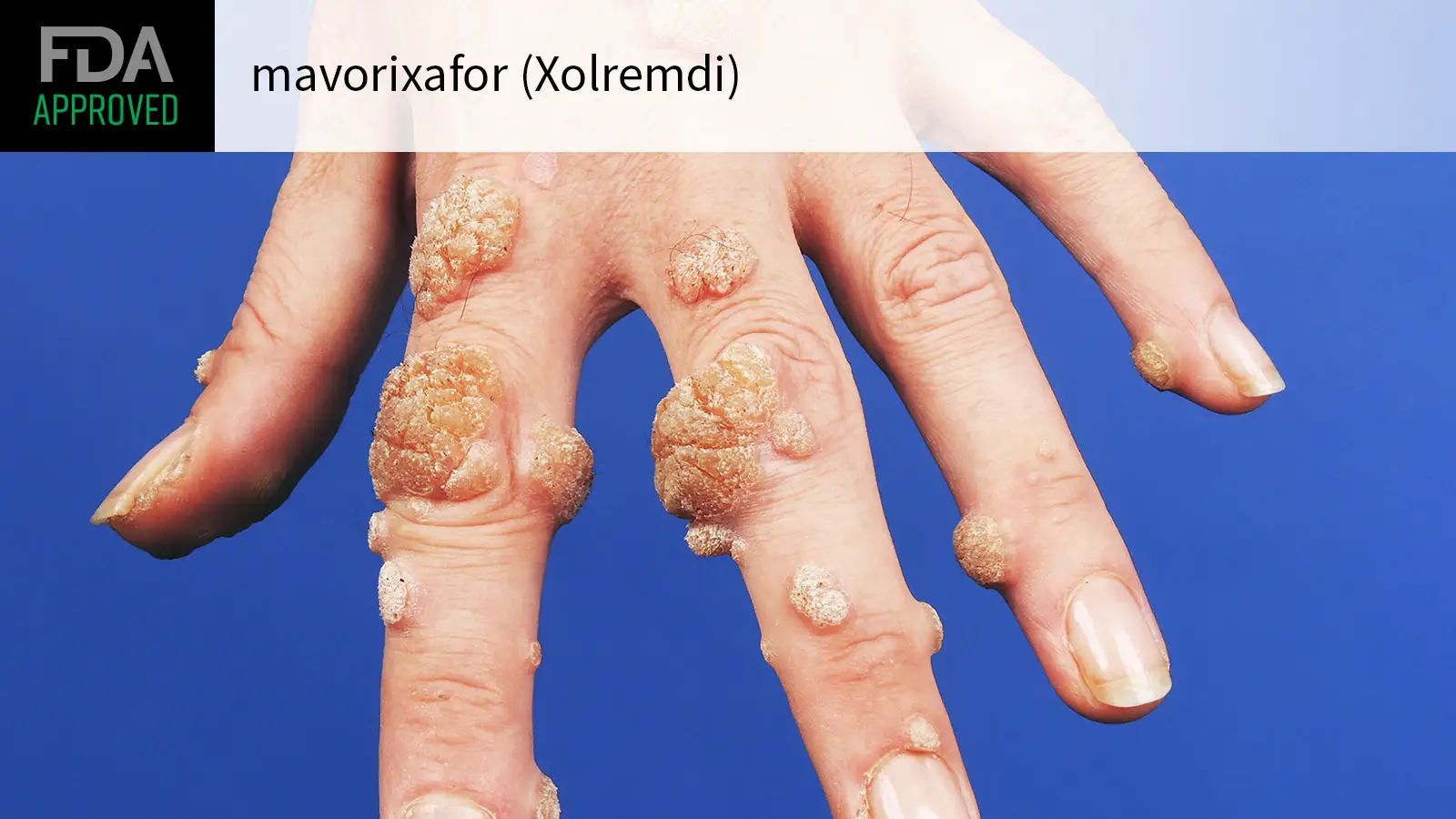
Following the licensing agreement with X4 Pharmaceuticals, Norgine is pleased to see the announcement from X4 that their Marketing Authorization Application (MAA) for mavorixafor for the treatment of WHIM syndrome (warts, hypogammaglobulinemia, infections and myelokathexis), a rare primary immunodeficiency, has been validated for review and is now under evaluation with the European Medicines Agency's (EMA) Committee for Medicinal Products for Human Use (CHMP). In April 2024, mavorixafor received U.S. Food and Drug Administration approval as XOLREMDI®, an oral, once-daily treatment for use in patients 12 years of age and older with WHIM syndrome.
Norgine is working with X4 to enable access to mavorixafor for patients in Europe, Australia and New Zealand and this regulatory milestone is an important step toward this goal.
© 2026 Biopharma Boardroom. All Rights Reserved.