10 July 2023 | Monday | News
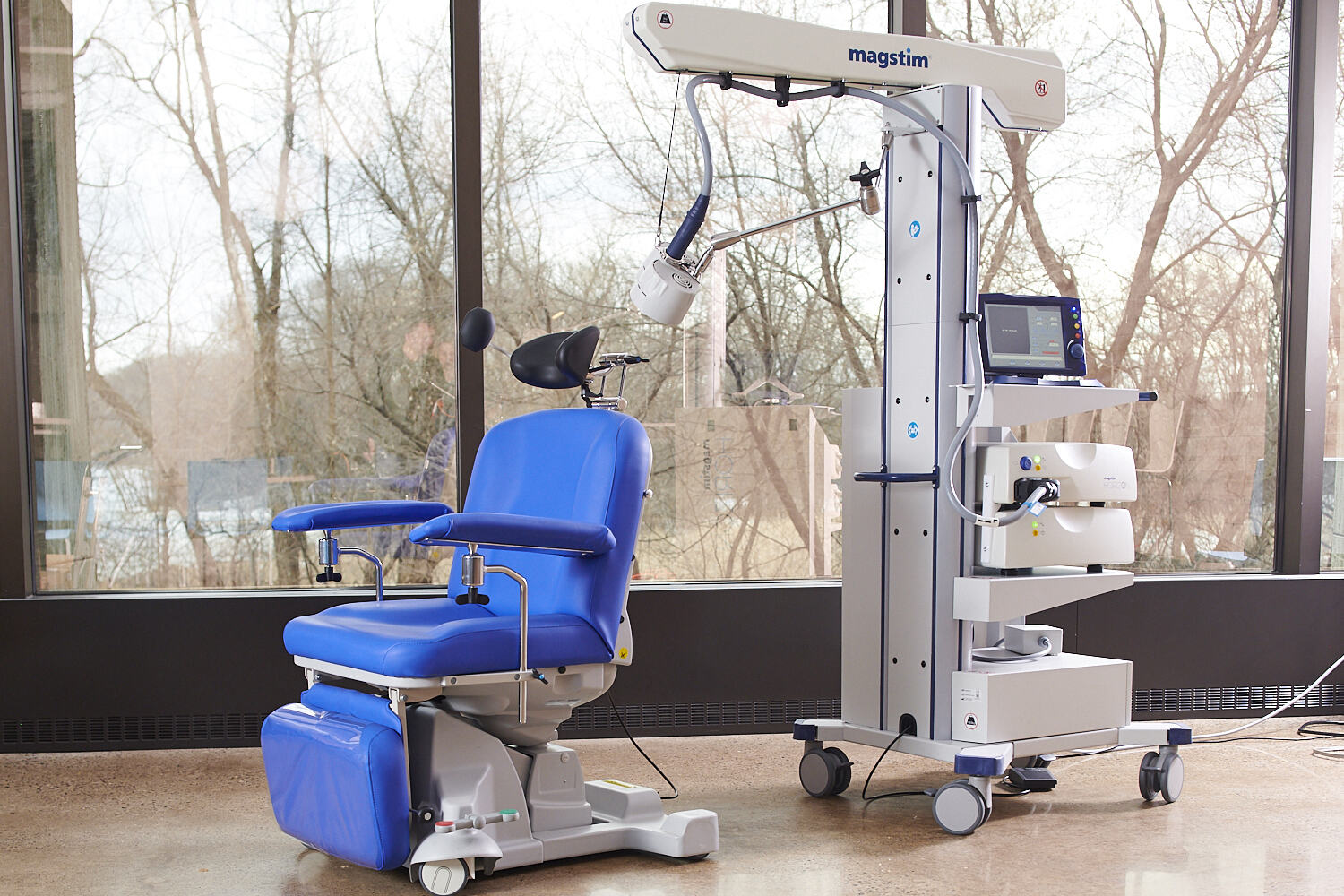
The advanced and FDA-cleared Magstim Horizon 3.0 transcranial magnetic stimulation (TMS) system is now UKCA registered and available for sale in the UK, providing physicians with a proven precision therapy to improve the lives of patients with depression, obsessive compulsive disorder (OCD), and anxious depression1.
The first company to manufacture and commercialize TMS as a treatment for brain disorders, Magstim technology has been cited in more than 16,000 published studies in the neuroscientific and clinical research.
“Magstim was founded in Wales, where our TMS line is manufactured and continues to be advanced,” said Ronnie Stolec-Campo, CEO, Magstim. “The Horizon 3.0 technology is already in use in the US, and we are thrilled and honoured to make this technology available to the dedicated UK physicians working hard to advance the field and alleviate the pain and suffering caused by mental illness.”
From July 10-13, Professor Alex O’Neill Kerr, MBChB, Medical Director of Transforming Mind Solutions, and the Magstim team will be at the Royal College of Psychiatrists Congress in Liverpool to educate clinicians on utilizing Horizon 3.0 in their practices. Studies show that patients respond positively to Magstim TMS Therapy2. Horizon 3.0 offers an intuitive system that optimizes treatment and helps improve patient care.
“Our focus is developing innovative treatments to aid the cure of depression and anxiety,” states Professor Alex O’Neill Kerr. “Our patients have achieved significant improvements in depression and mental health using TMS therapy. We use Magstim technology and look forward to this Horizon 3.0 launch in the UK.”
© 2026 Biopharma Boardroom. All Rights Reserved.