20 September 2024 | Friday | News
Picture Courtesy | Public Domain
Multi-center, multi-national study to evaluate mechanical thrombectomy treatment in acute, intermediate-risk PE patients in European Market
The RECOVER-AV trial is designed to evaluate the safety and efficacy of AlphaVac for the treatment of acute, intermediate-risk PE to support its adoption in the European market.
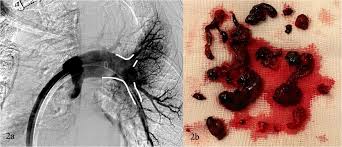
This study follows the Acute Pulmonary Extraction Trial with AlphaVac (APEX-AV) study, a single-arm Investigational Device Exemption study that enrolled 122 patients with confirmed acute, intermediate-risk PE across 25 hospital-based sites in the United States to assess the AlphaVac F18⁸⁵ System for the treatment of PE. APEX-AV, which completed enrollment in December 2023, demonstrated that the AlphaVac F1885 System is safe in patients with acute intermediate-risk PE and provides significant improvement in right ventricular function and reduction in clot burden.
The RECOVER-AV study is a multi-center, multi-national trial enrolling patients with confirmed acute, intermediate-risk PE across up to 20 hospital sites in Europe. The primary efficacy endpoint is the reduction of the right ventricular/left ventricular (RV/LV) ratio from baseline to 48 hours post-procedure. The primary safety endpoint focuses on the incidence of Major Adverse Events (MAEs), such as device-related death or major bleeding within seven days. Patients will be followed for 12-months, with functional outcomes assessed at 30-days, six-months, and 12-months.
An estimated 435,000 PE events occur each year in the six largest European Union (EU) countries.1 Compared to the United States, the prevalence of PE is higher for those patients admitted to the emergency department in Europe, and European patients also had higher acuity and worse outcomes.2
“We are excited to launch this important trial as we assess the performance of the AlphaVac F1885 System in patients with intermediate-risk pulmonary embolisms,” said Laura Piccinini, AngioDynamics Senior Vice President and General Manager of Endovascular Therapies and International. “With our clinical partners, we are demonstrating our continued commitment to generating robust clinical evidence across the world, with this being the first international study we have sponsored highlighting our commitment as a global leader to treat more patients and advance care.”
In May, the Company announced that its AlphaVac F1885 System received CE Mark approval in Europe for the non-surgical removal of thrombi or emboli from pulmonary arteries, including for treating PE. This approval allows the Company to expand its reach in the European market, where PE prevalence is notably higher than in the U.S.2 The AlphaVac F1885 System is designed to enhance treatment options for healthcare professionals and improve outcomes for patients suffering from PE.
The Study is led by co-Principal Investigators Erik Klok, MD, Professor of Medicine and Vascular Medicine Specialist at Leiden University Medical Center, and Andrew Sharp, MD, Professor of Interventional Cardiology at Mater Misericordiae Hospital and University College Dublin.
They are supported by an internationally renowned Scientific Advisory Board featuring leading experts in pulmonary embolism (PE) and related fields, including Prof. Menno Huisman (Leiden UMC, Netherlands), John Moriarty, MD (UCLA, USA), Prof. Makis Avgerinos (University of Athens, Greece), Prof. Irene Lang (Medical University of Vienna, Austria), Julie Helms, MD (CHRU Strasbourg, France), and Sebastian Stefaniak, MD, and Mateusz Puslecki, MD (PUMS, Poland).
© 2026 Biopharma Boardroom. All Rights Reserved.